YRGCARE was established in Chennai (1993) with an aim to improve awareness and spread prevention messages among the communities. Over the past 30 years, YRGCARE has provided clinical care for nearly 2 million people living with HIV and vulnerable populations. As of 2022, our services and reach are spread all across India. This has been made possible through association with the National AIDS Control Organisation (NACO), domestic / international partners, and well wishers.
Our services include counselling, testing, prevention, clinical care and treatment, state-of-the-art laboratory services, referrals / linkages, and allied services for dental, eye care. Many of our services are provided through our projects like ACCELERATE, Camp Rainbow, MOMENTUM Routine Immunization Transformation and Equity, GFATM, and more.
YRGCARE Services

Specialised HIV
Clinics

The YRGCARE Suniti
Solomon Outpatient Clinic

Well Women
Clinic

Pediatric
Clinic

Satellite Clinics

Integrated Care
Centres

Adolescent Friendly
Health Centres

Mitr Clinics
(for Trans-persons)
The YRGCARE Suniti Solomon
Outpatient Clinic
Our clinical services extend to people with all infectious diseases, with a focus on HIV, Hepatitis C,TB and non-communicable diseases in persons living with HIV. We use a hub-and-spoke model for the general population.

Highlights
- Outpatient Care for PLHIV including Women, Children and Adolescents
- Laboratory services for Testing, Care, and Treatment
- Subsidised pharmacy with adherence counselling
- Pre, post - test, and supportive counselling
- Nutritional assessment and counselling
- Marriage and fertility counselling
- Free services for children and adolescents
- Free health checkup and immunization for women

Our Clinic in Chennai serves as the hub connected to a network of hospitals (government/private) and centres of excellence within the city. We attend to patients with HIV admitted in any part of the city and offer pharmacy services at subsidised rates to patients across India.
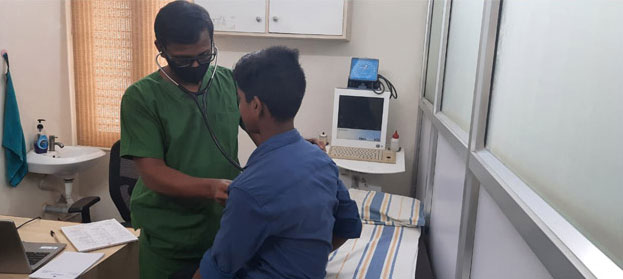
We also provide medical care, psychosocial support and skills development programs for children living with HIV. Children and adolescents are offered free ART, dental screening and visual aids with optometry apart from subsidised ART monitoring package (lab tests). We offer subsidised screening of cardiovascular health and link to care with the help of a paediatric interventional cardiologist.

Satellite Clinic
Our longest serving satellite clinic is in Andhra Pradesh, in collaboration with the Indian Red Cross Society. This Clinics is operational between Monday and Saturday from 9:30 AM to 5:30 PM. The clinic is equipped with a trained physician, nurse, counsellor, who are available for medical and psychosocial care. The clinic is equipped with a laboratory to conduct HIV spot tests, CD4 count, HIV/HCV viral load, biochemistry and hematology and a subsidised pharmacy.
YRGCARE
Infectious Diseases Laboratory
Established in 2000 , in collaboration with Johns Hopkins University, Baltimore, USA and Brown University, Rhode Island, USA with a financial grant from the National Institutes of Health (NIH), an agency of the United States Department of Health. Our laboratory with state-of-the-art equipment is staffed by experienced and qualified professionals, positioned to meet the emerging needs of HIV and other infectious diseases including sexually transmitted infections [STIs], tuberculosis [TB], COVID-19 and viral hepatitis. We conduct biomedical research in the areas of the basic sciences and offer educational programs organized by qualified scientists/faculty, and also a PhD program in Medical Microbiology.

Lab Divisions
Serology

Microbiology & Mycobacteriology

Biochemistry & Hematology

Immunology & Core Immunology

Molecular Biology & Genotyping
Pharmacology
We have adopted the ISO15189:2012 quality management system to provide the best world class diagnostic services to clients and patients attending YRGCARE clinics and for the samples referred from other parts of the country/world.
Our expertise ranges from support for clinical trials to seroepidemiological surveillance
- Programmatic support for HIV prevention among key populations.
- ART monitoring, bidirectional screening for TB, and screening for MDR TB.
- Whole genome sequencing for high impact pathogens with pandemic potential.
We constantly strive to offer accessible quality testing, and timely results.
Contact for more information: Mail info@yrgcare.org, Call 91 44 3312 5000


